Our Research
Research within the ATTACK project will elucidate the mechanisms of action by which cytotoxic T cells act against tumor cells in order to develop more targeted cancer treatments with less side effects for patients.
Our Research
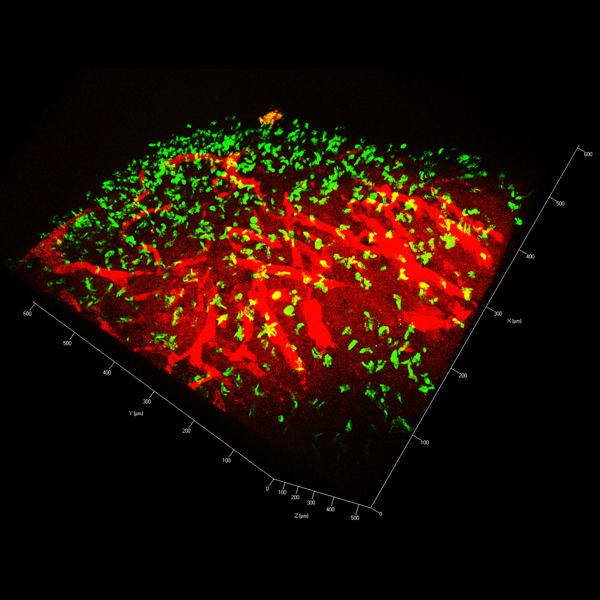
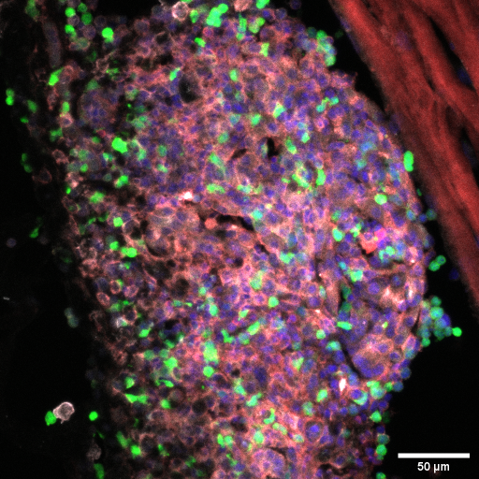
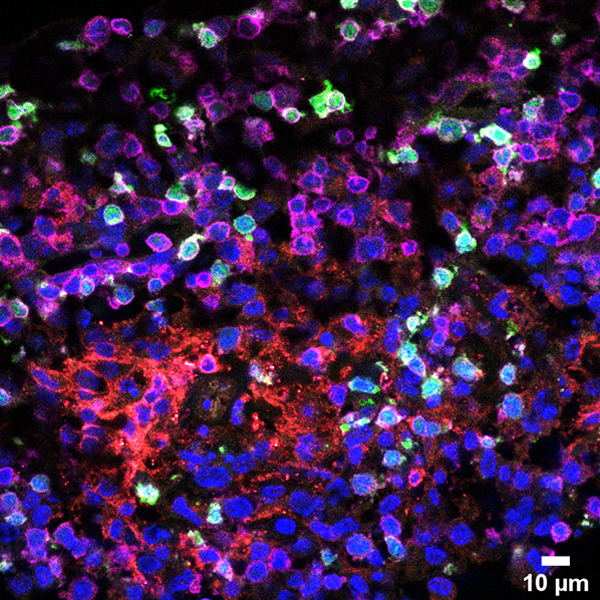
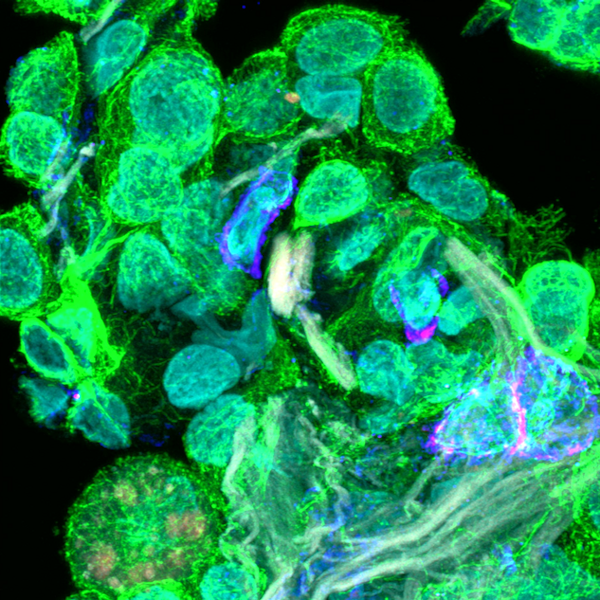
The ATTACK consortium will develop a new biotechnology based on harnessing a previously unknown natural cytotoxic mechanism of T cells to fight cancer. Cytotoxic T cells (CTL) protect us against intracellular pathogens and cancer by killing infected and cancerous cells.
It has been believed that CTL operate on two different time scales of killing by releasing soluble cytotoxic proteins from dense core granules into the immunological synapse between the T cell and target cells (seconds/minutes) and by FasL-mediated apoptosis (hours/days).
Members of the ATTACK consortium have independent observations that converge on a previously unknown weapon in the T cell tactical arsenal: stable supramolecular attack particles (SMAPs) that kill target cells (hours). This raises the possibility to engineer these particles to operate independent of T cells.
The consortium has expertise in mouse models, human immunology, gene editing in primary T cells, super-resolution and live cell microscopy, and microfluidics and will integrate all it learns to develop the biotechnology of SMAP enhancement in engineered T cells, recombinant SMAPs, and synthetic SMAPs. The vision is that SMAPs will be freeze-dried and shipped around the world, solving problems related to current immunotherapies, leading to global health impact.
Our Goals
The goal of the ATTACK consortium will be to work closely together through 4 work packages to determine
Work Packages
Work package 1
Leading Principle Investigator: Cosima Baldari, University of Siena
Aim 1: Dissecting the trafficking pathways responsible for the transport of the SMAP components.
Aim 2: Engineering HEK293 cells as a bioreactor for the efficient production of SMAPs.
Aim 3: Engineering SMAPs with specificity for CLL B cells.
Aim 4: Testing the CLL killing ability of SMAPs ex vivo and in vivo.
The results will provide new knowledge on the traffic pathways that regulate the biogenesis of DCG, MCGs and SMAPs, also contributing to clarify the relationships among these lytic particles (Aims 1 and 2). Additionally, they will set the basis for engineering HEK293 cells to produce functional target-specific SMAPs that can be used for combination therapies in CLL (Aims 2-4). The data will provide proof-of-concept for the therapeutic potential of targeted SMAPs for other cancers.
Work package 2
Leading Principle Investigator: Jens Rettig, Saarland University
Aim 1: Mechanism of DCG and MCG fusion.
Aim 2: Contribution of Ca2+ and pH to SMAP release.
Aim 3: Determining the composition of fusogenic granules in primary human CTL.
Aim 4:Analysing SMAP activity in an in vivo model.
Careful analysis of MCG composition and physical characteristics will be critical to the overarching goals of the ATTACK consortium. We will deliver critical information in the proteins and lipids bound in the MCG, as well as the pH and [Ca2+] in the granule, which will be critical for understanding the conformation and potential binding partners of TSP-1 in the human system. We will identify candidates for the TSP-1 counterpart in mouse CTL. We will deliver in vitro data on release kinetics of soluble and SMAP associated GzmB. We will also deliver in vivo data on the fate of SMAPs in the tumour microenvironment.
Work package 3
Leading Principle Investigator: Michael Dustin, University of Oxford
Aim 1: Determine the composition and structure of SMAPs.
Aim 2: Identify key SMAP ligands.
Aim 3: Determine the activation mechanism of SMAPs.
Aim 4: Bottom up assembly of synthetic SMAPs.
We will deliver resources reporting the composition, heterogeneity and structure of SMAPs across human and mouse CTL and NK cell types. We will further define the specific receptors interactions available to SMAPs and whether they target particular proteins, lipid, or carbohydrate components on the surface of tumour cells. Finally, we will study the activation mechanism of SMAPs to determine if this can be tuned to generate longer range SMAPs that are stable enough to be used as systemic therapeutics. We will further deliver fully tested synthetic SMAPs, which will be the product of information from across the ATTACK consortium.
Work package 4
Leading Principle Investigator: Salvatore Valitutti, Inserm Cancer Research Centre Toulouse
Aim 1: SMAPs in human antigen specific CTL killing strategies.
Aim 2: The SMAPs from the tumour cell point of view.
Aim 3: The SMAPs for human CTL and CAR-T cell optimisation.
The research we propose is expected to provide results relevant to:
- The hierarchy of soluble GzmB/Prf/Srgn complexes and SMAP release at the IS in terms of amount, timing and efficacy;
- The functional impact of SMAPs in specific and bystander killing;
- Mechanisms and counter measures for tumour cell resilience to SMAPs relative to classical killing mechanisms;
- Strategies to re-invigorate CTL and CART cells or replace them with cell-free ExoSMAPs.